OPA’s Influenza Vaccines Education Programs
We have been seeing an increase in public demand for receiving their flu shot at pharmacies in the past few years. Pharmacies are accessible and have the training to effectively and efficiently roll out large immunization campaigns. During the 2021 flu season, community pharmacies in Ontario were responsible for administering more than 1.8 million flu shots.
The Ontario Pharmacists Association, in partnership with Seqirus, has developed and designed several resources, as well as education, live, and on-demand programs to support pharmacy professionals in successfully operationalizing influenza vaccines in their pharmacies.
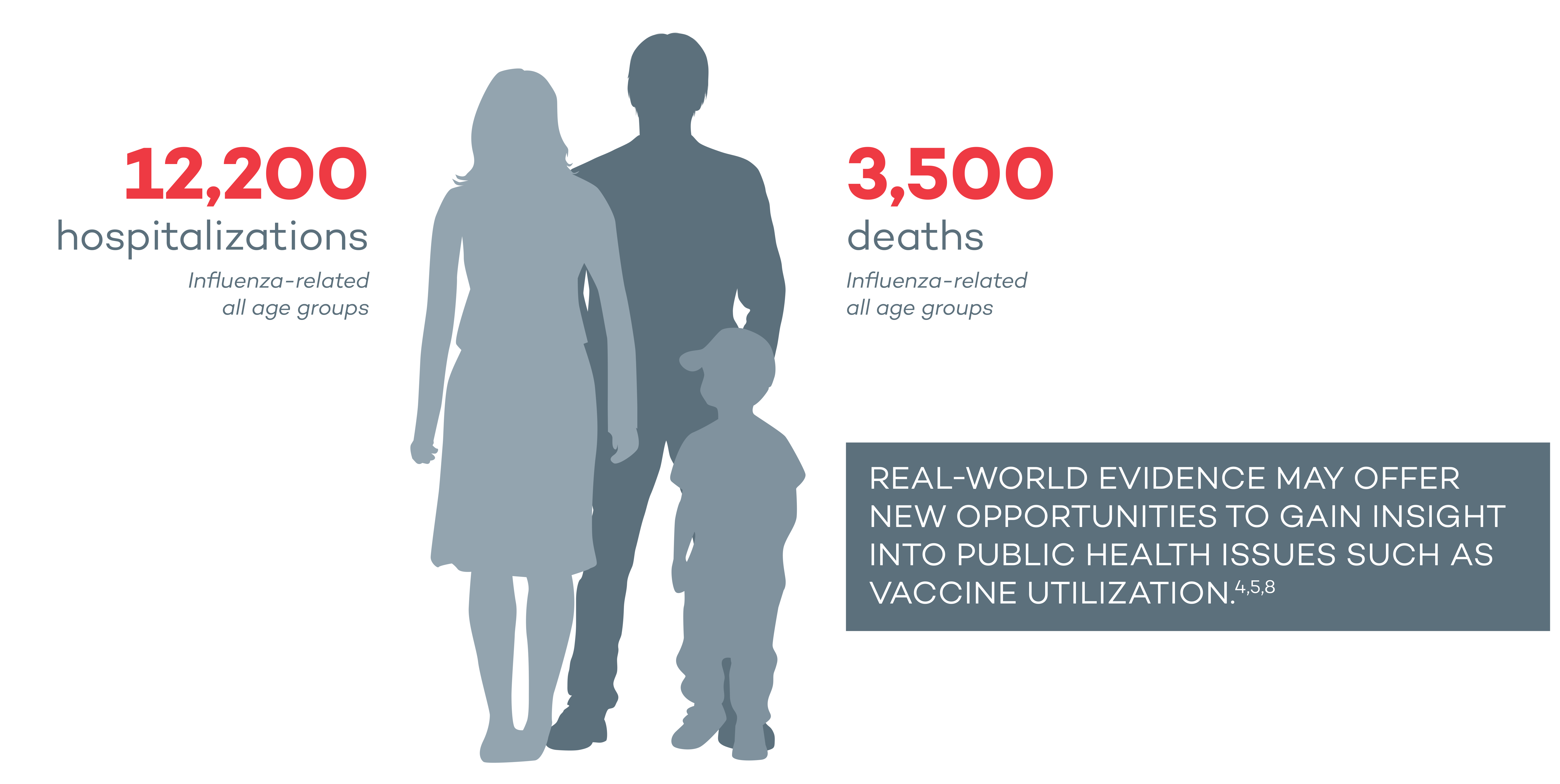
Influenza Has Impacted the Lives of Many Canadians
Influenza has been reported to have a high burden in hospitalizations and mortality.2*
According to NACI 2020-2021, together, influenza and pneumonia are ranked among the top 10 leading causes of death in Canada.2
*Influenza vaccines are not indicated to reduce morbidity or mortality following the onset of influenza.
Influenza Viruses and the Ever-Present Possibility of Antigenic Drift 1,2
Many factors may reduce influenza vaccine effectiveness2*
- Strain mismatch between the vaccine strains and the circulating influenza viruses
- Type and subtype of circulating viruses
- Health and age of the individual receiving the vaccine
*Not an exhaustive list.
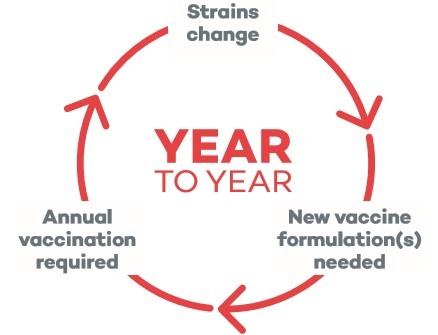
Vaccinating Against Influenza Is Complex 1,2
- Influenza virus strains may change from year to year due to antigenic drift
- Annual reformulation is needed
- Vaccine effectiveness can vary
Real-world evidence contributes valuable information regarding influenza vaccines and their use to help guide decision-makers4-7
- Based on real-world data which is timely and increasingly available
- Complements traditional randomized-controlled trials
- Can reflect the dynamics of actual healthcare systems
The influenza virus is the only pathogen that we vaccinate for annually.
Because the circulating strains of influenza virus may change from year to year, annual reformulation and revaccination are required to protect the public against influenza.1,2 This means we need to evaluate influenza vaccines on an annual basis.3
Live Webinar: Existing and Novel Approaches in Vaccine Science
Injections and Immunizations Refresher Program
Live Webinar: Get Ready for Flu Shots
References:
Government of Canada. Vaccination for adults. https://www.canada.ca/en/public-health/services/vaccination-adults.html#a5. Published March 29, 2019. Accessed on September 13, 2020.
Public Health Agency of Canada. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2020–2021. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2020-2021.html. Published May 2020. Accessed on September 13, 2020.
Government of Canada. Access to the seasonal flu vaccine in Canada. How the flu shot makes its way from the laboratory to the doctor’s office. http://publications.gc.ca/collections/collection_2007/hc-sc/H164-47-2007E.pdf. Published 2007. Accessed on September 13, 2020.
Government of Canada. Elements of Real World Data/Evidence Quality throughout the Prescription Drug Product Life Cycle. https://www.canada.ca/en/services/health/publications/drugs-health-products/real-world-data-evidence-drug-lifecycle-report.html. Published March 5, 2019. Accessed on September 13, 2020.
Government of Canada. Optimizing the Use of Real World Evidence to Inform Regulatory Decision Making. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html. Published April 16, 2019. Accessed on September 14, 2020.
The National Academies of Sciences Engineering Medicine. Real-World Evidence Generation and Evaluation of Therapeutics: Proceedings of a Workshop. 2017. https://www.nap.edu/catalog/24685/real-world-evidence-generation-and-evaluation-of-therapeutics-proceedings-of (accessed Nov. 4, 2020).
FDA. Framework for Real-world evidence program. 2018.
PHAC. Seasonal influenza vaccine coverage in Canada. 2017-2018.
